An application of the first law of thermodynamics to a process in which any work terms are negligible.
For a closed system, one that always consists of the same material, the first law is Q + W = ΔE, where Q is the heat supplied to the system, W is the work done on the system, and ΔE is the increase in energy of the material forming the system. It is convenient to treat ΔE as the sum of changes in mechanical energy, such as kinetic energy and potential energy in a gravitational field, and of internal energy ΔU that depends on changes in the thermodynamic state of the material. Because the rates at which any changes occur are usually of interest, heat balances are often written in terms of heat flow rates (heat per unit time), sometimes denoted by a dot over the symbol, , so that for a process with negligible work, kinetic energy and potential energy terms, , the rate of change of internal energy with time.
Often it is more convenient to apply the first law or a heat balance to an open system, a fixed region or control volume across the boundaries of which materials may travel and inside which they may accumulate, such as a building, an aircraft engine, or a section of a chemical process plant. Then the first law is expressed by the equation below, where is the rate of doing shaft work
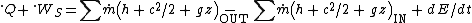
Enthalpy is a thermodynamic property defined by h = u + pv, where u is the specific internal energy (enthalpy per unit mass), p the pressure, and v the specific volume. It is used, along with shaft work, because the derivation of the first-law equation for a control volume from the more fundamental equation for a closed system involves work terms pv that are not available for use outside the control volume. Changes in enthalpy occur because of changes in temperature, pressure, physical state (for example, from liquid to vapor), and changes in chemical state
