Heat engine is defined as
a device that converts heat energy into mechanical energy or more exactly
a system which operates continuously and only heatand work may pass across its boundaries.
The operation of a heat engine can best be represented by a thermodynamic cycle. Some examples are: Otto,
Diesel, Brayton, Stirling and Rankine cycles.
Forward Heat Engine
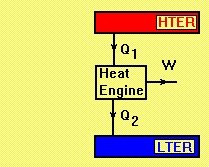 LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
A forward heat engine has a positive work output such as Rankine or Brayton cycle. Applying the first law of thermodynamics to the cycle gives:
Q1 - Q2 - W = 0
The second law of thermodynamics states that the thermal efficiency of the cycle,

, has an upper limit (the thermal efficiency of the Carnot cycle), i.e.

It can be shown that:
Q1 > W
which means that it is impossible to convert the whole heat input to work and
Q2 > 0
which means that a minimum of heat supply to the cold reservoir is necessary.
Reverse Heat Engine
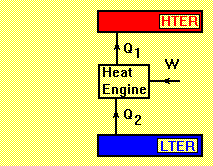 LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
A reverse heat engine has a positive work input such as heat pump and refrigerator. Applying the first law of thermodynamics to the cycle gives:
- Q1 + Q2 + W = 0
In case of a reverse heat engine the second law of thermodynamics is as follows: It is impossible to transfer heat from a cooler body to a hotter body without any work input i.e.
W > 0
which means that the coefficient of performance for a heat pump is greater than unity.