Currently, different handbooks used in science and technology are being transferred from “paper” carriers to Internet sites. At present, if you need the value of heat conductivity of brass at a certain temperature, it is easier to type the key words “heat conductivity,” “brass,” and “temperature” into the entry window of a search engine (www.yandex.ru, www.google.ru, http://search.msn.com, etc) rather than browse through a voluminous book. However, new problems (and solutions of them!) of a special kind are emerging, and they are considered in this study.
The reliability of the information contained in “paper” handbooks is to some extent ensured by the reputation of the corresponding publishing houses and their staff of scientific consultants, editors, and correctors. Internet sites are, as a rule, created by nonprofessional developers and their content is not subject to strict editing and careful proofreading procedures. Nevertheless, I have found a comparatively large number of misprints in handbooks published by authoritative publishing houses. For example, 0.02387 maybe printed instead of 0.02387 (i.e., the digit 2 denoting the number of repeated zeros is misused), or 72.93 instead of 27.39 (in this case, the number was probably typed by a German-speaking person, in whose language “twenty-seven” is “seven and twenty” (siebenundzwanzig) and “thirty-nine” is “nine and thirty” (neununddreissig)), etc. Such typos may remain unnoticed in the process of traditional (visual) proofreading of “paper” handbooks, and, as a result, the book appears with a list of misprints (Errata – see one example >>>>>>>). In brief, misprints remain misprints with all the ensuing consequences. Reportedly, Academician A.N. Krylov (1863-1945) applied while a student for a position in an engineering bureau. As a test, he was asked to go over a bridge-building project. The future academician started to examine the project and soon exclaimed, “It is not possible to erect such a bridge: it will collapse!” The answer was: “That’s true; it has been built and has collapsed. You are hired!” Perhaps the future expert in mechanical science found a typo in a reference table used in the bridge project similar to those described above? The instrumental means considered below allow timely identification of such typos in reference data or, at least, reduction of negative consequences deriving from them.
Recently, tools for publishing documents on the Web (on the Internet or corporative networks) created using applied mathematical software have been multiplied greatly. In the case of Mathcad [1 – 4], the Mathcad Application Server [4 – 6] is the kit that is used for this purpose. However, one can publish on the web, apart from pure calculation (examples of such calculations for thermal power engineering are posted at www.vpu.ru/mas), hybrid forms consisting of tables, plots, formulas (the dominant of “paper” reference information), and calculations. The instruments built into these mathematical packets make it possible to carry out statistical processing of tabular data and display the requested information in an “intelligent” form. For example, having opened the page of a “paper” handbook [7] containing information about the heat conductivity of brass, one will see a table the side column of which contains a list of alloys, including brasses of different composition, and a heading with the values o temperature for which the values of heat conductivity are displayed in the table. If one visits the Internet site located at http://twt.mpei.ac.ru/MAS/Worksheets/Therm/Heat_Cond_metal_e.mcd, one will see the data shown in Fig. 1.
A visitor to this website may select the alloy he is interested in the list, enter the value of temperature (in different scales – Celsius, Kelvin, Fahrenheit, or Rankine), and obtain the required value of heat conductivity (also in various units). The system also displays plots showing dependence of heat conductivity on temperature, which makes it possible to study the required quantity as a function of temperature and see the current point on a curve. Moreover, a visitor to the website may set the power n of the polynomial approximating the tabular data an output its coefficients for subsequent use of the displayed dependence in other applications, for example Excel broadsheets. Figure 2 shows how the coefficients of the approximating polynomial of the third power are entered (copied from the web page shown in Fig. 1) into an Excel formula field (cell B3) to calculate the value of specific heat conductivity for the value of temperature contained in cell B2. The reader can easily understand that these coefficients may be copied as well into the fields of programs newly developed or edited in BASIC, Pascal, C, Fortran, etc. Figure 1 displays two plots: a spline interpolation (upper curve) and approximation (lower curve). This is done intentionally to provide more information and the option of choice to a visitor to the site. In addition, this duality may be considered a significant characteristic of the website. The point here is that the interpolation procedure (when the curve exactly passes through the points not displayed in the first plot) makes it possible to clearly identify misprints that were contained in the original tables or were made while transferring data from “paper” source to a computer by scanning or typing. At the same time, the approximation procedure (when the curve passes in the vicinity of the points that are displayed in the second plot) makes it possible to minimize the consequences of such misprints if they have not been identified by means of interpolation. Reference information also includes different formulas needed for calculations. In this case, the Mathcad Application Server may also prove to be very useful for publishing formulas on the Web.
Figure 3 shows as an example a web page containing four formulas. These expressions describe a change in temperature in a spherical wall under the conditions of steady heat transfer and with the heat conductivity of the wall material not depending on temperature (a simplified problem). The formulas are “animated”, i.e., the visitor can “play” with the variables: change the initial data and see new values retrieved by the formulas and the corresponding point on the plot, which also changes depending on the initial data. “Animation of formulas serves two purposes. First, the visitor can immediately obtain a result that follows from the formulas without entering them into his computer or calculator. Second, this provides an extra option to check whether the formula on the “paper” contained an error or the formula was incorrectly typed into computer when creating respective Mathcad document posted on the Web using the technology of the Mathcad Application Server. The Mathcad package, which contains a mechanism for checking dimensions [8], significantly reduces the probability of such errors. The Web page may contain a hyperlink to a scanned “paper” page of the source, for example, a handbook or even an experiment log (see the penultimate line in Fig. 3).
The formulas contained in the reference Web pages may be transferred to other program environments either manually, in the visual mode, or automatically. The most recent versions of the package, Mathcad 12 [4] and Mathcad 13, support recording of files used by this mathematical package in HTML format (hyper text markup language). Therefore, it is now possible to view and edit Mathcad files on a PC without using Mathcad itself. Such a file posted on the Web for downloading (see the link in the lower part of Fig. 3) may be opened by any word processor. In this case, a reference formula presented in text format may be copied to any program environment, for example, Maple (fig. 4), where the formula will be converted into a graphical form more convenient for visual inspection.
The list of typos usually present in each handbook often contains as well information about typos in formulas. This is a consequence of the formulas being prepared for publication using equation editors, such as MS Equation, rather than mathematical packages that allow testing of their “operability.”
Implementation of reference in the references on the Web using instruments of mathematical packets and their tools for graphic visualization makes it possible to represent information about the functions of two or more arguments in an innovative form. The web page shown in Fig. 5 displays not only values of entropy s, specific volume v, and enthalpy h of water or steam in different units for the parameters specified by site the visitor (steam pressure and temperature), but also the respective isotherm and isobar on the respective thermodynamic surface with saturation lines for water and steam (see also www.wsp.ru, the website of the package WaterSteamPro [9], the functions of which were used for plotting the thermodynamic surface shown in Fig. 5).
I have developed templates of Mathcad documents for Web “animation” of various data: fully populated tables, scattered tables with shifted arguments or diffused ranges, etc. [4]. The main amount of labor is needed in this activity to digitize tables. I was assisted by the students attending the course on information processing given at the Institute of Thermal Power Engineering and Engineering Physics of Moscow Power Institute. “Outlines” of popular scientific and engineering handbooks, textbooks, training aids, and other sources of information are gradually being formed on the Internet [10, 11]. For example, Fig. 6 shows a web page of an “animated” electronic version of a textbook by V.Ya. Rotach, Theory of Automatic Control (http://twt.mpei.ru/MAS/Worksheets/Rotach/index.html). The calculations presented in Rotach’s textbook are performed using the Mathcad environment. The reader can download the calculation files from the site (see the third row in Fig. 6). Alternatively, he can handle them interactively by changing the initial data and obtaining the result without installing on his computer additional software, an operation that can be either forbiddingly expensive or involve violation of license agreements.
The field of Internet handbooks based on mathematical programs makes it possible to easily implement requests related not only to separate points, i.e., fixed states of materials and coolants (see Figs. 1 – 5), but to entire processes. For example, Fig. 7 shows the page of a Web-based reference (www.vpu.ru/mas) displaying the process of isoentropic steam expansion, with the isotherm, isobar, and other curves that characterize this process being displayed on the h,s chart as well. This website also offers calculations and displays on the process charts of different expansion processes specific to actual steam-turbine engines, gas-turbine engines, combined cycle turbines, and “classical” cycles (Carno, Otto, Diesel, etc.). Different sets of variables (h and s, t and s, p and v, etc.) are supported.
Currently, some books are in the process of being digitized (see www.vpu.ru [12], www.thermal.ru [13], http://twt.mpei.ac.ru/GDHB [14], etc.). The Moscow Power Institute publishing house is now preparing for publication the fifth (additional) volume of handbook [7], containing a description of methods that can be used for creation of Web-based interactive tabled, plots, and formulas. Some chapters of this volume are already posted on the Internet at http://twt.mpei.ac.ru/TTHB.
REFERENCES
1. V.F. Ochkov, V.F. Utenkov, and K.A. Orlov, “Thermal Engineering Calculations in the Mathcad Environment,” Teploenergetika, No. 2, 73-78 (2000) [Thermal Engineering 47 (2), 173-180 (2000)].
2. V.F. Ochkov, A.P. Pil’shchikov, et al., “Analysis of Ion-Exchange Isotherms Using the Mathcad Software Package,” Teploenergetika, No. 7, 13-18 (2003) [Thermal Engineering 50 (7), 537-543 (2003)].
3. V.F. Ochkov, A.P. Pil’shchikov, and Yu. V. Chudova, “Open Calculations in Thermal Power Engineering,” Energosberezheniye I Vodopodgotovka, No. 1, 21-24 (2002).
4. V.F. Ochkov, Mathcad 12 for Students and Engineers (BKhV, St Petersburg, 2005) [in Russian]. 5. V.F. Ochkov, “Mathcad: from Plot to Formula, from Computer Calculation to Internet Calculation,” Exponenta Pro. Matematika v Prilozheniyakh, No.4, 84-85 (2003).
6. V.F. Ochkov, “Mathematical Packages: from Natural Economy to Production of Computer Commodities via Internet,” ComputerPress, No. 5, 172-173 (2004).
7. Theoretical Foundations of Thermal Engineering. Thermal Engineering Experiment. Handbook, Ed. by A.V. Klimenko and V.M. Zorin (Publishing House of the Moscow Power Institute, Moscow, 2001), 3rd revised and extended edition [in Russian]. 8. V.F. Ochkov, Physical and Economic Quantities in Mathcad and Maple (Finansy i Statistika, Moscow, 2002) [in Russian]. 9. A.A. Alexandrov, K.A. Orlov, and V.F. Ochkov, “Study of the Schemes for a Combined-Cycle Plant with Steam Injection into the Gas Path on the Basis of Development Applied Programs for the Properties of Working Fluids in Combine-Cycle Plants,” Novoe v Rossiiskoi Elektroenergetike, No. 4, 27-31 (2004).
10. V.F. Ochkov, O.G. Osipov, and M.V. Volokitin, “New Approaches to Publication in Industry Standards and Other Regulatory Documents Containing Calculations in a Power Engineering Corporate Network,” Novoe v Rossiiskoi Elektroenergetike, No. 10, 21-25 (2005).
11. V.F. Ochkov, “Thermal Engineering References in Internet,” Novoe v Rossiiskoi Elektroenergetike, No. 4, 48-58 (2005).
12. A.S. Kopylov, V.M. Lavygin, and V.F. Ochkov, Water Trearment in Power Engineering (Publishing House of the Moscow Power Institute, Moscow, 2003) [in Russian]. 13. A. Solodov and V. Ochkov, Differential Models. An introduction with Mathcad {Springer-Verlag, 2004). 14. V.F. Kasilov, Handbook on Gas Dynamics for Specialists in Thermal Power Engineering (Publishing House of the Moscow Power Institute, Moscow, 2000) [in Russian].







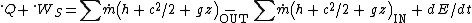



 = 10, this can be simplified to:
= 10, this can be simplified to: 

















