Heat Transfer series looks at the research and development in thermal engineering for power systems that are of significant importance to many scientists who work in power-related industries and laboratories. To be competitive in today's market, the editors say, power systems need to reduce operating costs, increase capacity, and deal with many other tough issues. Among the topics the book covers are: relevance of heat transfer and heat exchangers for development of sustainable energy systems; advanced technologies for clean and efficient energy conversion in power systems; virtual engineering and the design of power systems; and innovative gas turbine cooling techniques.
Thursday, February 17, 2011
Aluminum Fabrications
 Aluminium fabrication is a challenging task indeed. The special properties of aluminium often pose some serious challenges even to the most experienced welders. For example, aluminum is a very well conductor of heat. At the same time, it has a low melting point. Naturally, it becomes difficult to weld the aluminium sheet without burning at least some portions of it. Also, there are some impurities involved in the aluminium which is important to be got rid of. At the same time, one should preheat the aluminium sheet to ensure that the welding is done properly without any chance of cracks.
Aluminium fabrication is a challenging task indeed. The special properties of aluminium often pose some serious challenges even to the most experienced welders. For example, aluminum is a very well conductor of heat. At the same time, it has a low melting point. Naturally, it becomes difficult to weld the aluminium sheet without burning at least some portions of it. Also, there are some impurities involved in the aluminium which is important to be got rid of. At the same time, one should preheat the aluminium sheet to ensure that the welding is done properly without any chance of cracks.Regarding all these actions, the push technique is often considered preferable for the experienced professionals. This is basically the technique pushing the gun away from the welding puddle instead of pulling it towards it. The process ensures better cleaning actions, improved shielding gas coverage and reduced weld contamination. Anyways, it is important that the entire welding is done quickly. Since the thermal conductivity of aluminum is high, you need to use higher voltage and amperage settings. Also, you have to have high weld travel speed to ensure that the work is done during the right temperature.
When it comes to shielding gas for aluminum welding, the argon is the most popular option. This is largely because of its ability to clean and penetrate. However, if someone is welding 5XXX series of aluminum alloys, the use of a mixture of argon and helium is preferred. This will reduce the formation of magnesium oxide in the welding process.
Another tricky task for aluminum welding is to select the welding filler wire. Ideally, you should pick up a wire that has a very similar melting point to the base material. This is crucial, the narrower the gap between the filler and the base material, the easier the process will be. Also, you should prefer a wire with a large diameter.
One of the greatest challenges with the aluminum welding is the crater cracking. In fact, this is the most common factors that cause failure in aluminum welding. The crack occurs largely because of the higher rate of thermal expansion of aluminum. However, the risk is even greater when you are welding the concave craters as their surface tears at the time of contraction. Therefore, the welder must ensure that a convex or mound shape is developed during the welding. This will compensate the amount of contraction at the time of cooling down. Finally, you should always choose the power source tool according to the method of transfer spray arc.
Labels:
Aluminum Fabrications
Types Of Pressure Vessels
When a container is pressurized then pressure is exerted against the walls of the vessels. Pressure is always normal to the surface regardless of the shape. Pressure vessel is a container that has pressure different from the atmospheric pressure. There are many types of pressure vessel they are; thin walled, thick walled, strong tanks, transportable containers, propane bottles and gas cylinders. Pressure vessel is a container that holds liquid, vapor or gas at different pressure other then atmospheric pressure at the same elevation. Generally, a pressure vessel is considered to be thin-walled if its radius is larger than 5 times its wall thickness. Under this condition, the stress in the wall may be considered uniform. Thin wall pressure vessels are in fairly common use. There are two specific types of pressure vessels they are; cylindrical pressure vessels and spherical pressure vessels. Under this condition, the stress in the wall may be considered uniform. The stress in thin walled vessel varies from a maximum value at the inside surface to a minimum value at the outside surface of the vessel. Storage tanks are a category of thin walled pressure vessel.
A thick walled pressure vessel is the one that its wall is 10 % thicker than inside diameter. Thick walled pressure elements working in high temperatures in power stations, chemical and petro chemical industries are subjected to damage as a result of high temperature, mechanical loading and corrosive environment. These factors cause thermal fatigue, creep-fatigue and other processes leading to degradation. When subject to internal and external pressure stress and strain exist in thick walled pressure vessel. They have high tensile strength and can withstand maximum stress.
Transportable containers are the most common pressure vessel and potentially the most ignored type. These are mass produced and require testing every 10 years for propane and gas. Steel pressure vessels are designed to perform the dual purposes air storage and partial separation of moisture. These are provided with all standards so as to operate safely. Pressure vessels have to be designed to perform efficiently at high pressure. Pressure vessel must be free of any cracks or leakages, thereby ensuring complete safety to the surrounding environment. Cracked and damaged vessel may result in leaks or rupture failure. Rupture failure may cause considerable damage to life and property. The safe design, installation, operation and maintenance of pressure vessel in accordance with appropriate codes and standards are necessary for workers safety and health.
Labels:
Types Of Pressure Vessels
Thursday, January 27, 2011
Thermodynamics: Basic Terms
Thermodynamics: The branch of science that deals with the study of different forms of energy and the quantitative relationships between them.
System: Quantity of matter or a region of space which is under consideration in the analysis of a problem.
Surroundings: Anything outside the thermodynamic system is called the surroundings. The system is separated from the surroundings by the boundary. The boundary may be either fixed or moving.
Closed system: There is no mass transfer across the system boundary. Energy transfer may be there.
Open system: There may be both matter and energy transfer across the boundary of the system.
Isolated system: There is neither matter nor energy transfer across the boundary of the system.
State of the system and state variable: The state of a system means the conditions of the system. It is described in terms of certain observable properties which are called the state variables, for example, temperature (t), pressure (p), and volume (v).
State function: A physical quantity is a state function in the change in its value during the process depends only upon the initial state and final state of the system and does not depend on the path by which the change has been brought about.
Macroscopic system and its properties: If as system contains a large number of chemical species such as atoms, ions, and molecules, it is called macroscopic system. Extensive properties: These properties depend upon the quantity of matter contained in the system. Examples are; mass, volume, heat capacity, internal energy, enthalpy, entropy, Gibb's free energy. Intensive properties: These properties depend only upon the amount of the substance present in the system, for example, temperature, refractive index, density, surface tension, specific heat, freezing point, and boiling point.
Types of thermodynamic processes: We say that a thermodynamic process has occurred when the system changes from one state (initial) to another state (final).
Isothermal process: When the temperature of a system remains constant during a process, we call it isothermal. Heat may flow in or out of the system during an isothermal process.
Adiabatic process: No heat can flow from the system to the surroundings or vice versa.
Isochoric process: It is a process during which the volume of the system is kept constant.
Isobaric process: It is a process during which the pressure of the system is kept constant.
Reversible processes: A process which is carried out infinitesimally slowly so that all changes occurring in the direct process can be exactly reversed and the system remains almost in a state of equilibrium with the surroundings at every stage of the process.
Labels:
Thermodynamics Basic Terms
Saturday, January 15, 2011
What Goes Around Comes Around Thermal
I’ve spent a lifetime committed to helping others. It’s what gives me the greatest joy, fulfillment and satisfaction in life. I’m also a true believer in the old adage - “What goes around comes around.”
This is reinforced on many occasions when I encounter an old co-worker, friend or acquaintance who reminds me of how I inspired them, gave them guidance or helped them through a difficult time. These kinds of experiences always motivate me to keep putting good things out into the world.
We never know in advance what good may come from the gestures we put out there. Maybe it will inspire, encourage, educate, motivate or actually change someone’s life. In any event, people will always come away knowing that they matter to you.
In business, as in life, it all comes down to people. People are your customers, co-workers, employees, leaders, managers, investors and vendors. Success in your business depends on the people you encounter. How you treat those people will have a significant impact on your bottom line.
Here are some ways to help people in your business and show them that they matter:
1. Deliver incredible customer service.
2. Give a single parent the afternoon off.
3. Pay someone more than you need to.
4. Send a hand-written note to an employee acknowledging a job well done.
5. Loan a subordinate a book that you’ve found to be helpful.
6. Tell someone struggling “I want to help you succeed,” and mean it.
7. Stop by and talk, without an agenda.
8. Share a resource that you’ve found useful.
9. Create a safe space for openness and honesty.
10. Let people tell you how they feel, and listen without interrupting.
I encourage you to think about the people that you impact, the lives that you touch and the positive changes that you can make. These are the true signs of leadership and in my opinion are more important than the money that you make, the position that you hold or the size of your office.
You can do good while you’re doing well. It may require you to think of new ways to do it, but when you focus on helping others, you’ll succeed in ways beyond measure.
This is reinforced on many occasions when I encounter an old co-worker, friend or acquaintance who reminds me of how I inspired them, gave them guidance or helped them through a difficult time. These kinds of experiences always motivate me to keep putting good things out into the world.
We never know in advance what good may come from the gestures we put out there. Maybe it will inspire, encourage, educate, motivate or actually change someone’s life. In any event, people will always come away knowing that they matter to you.
In business, as in life, it all comes down to people. People are your customers, co-workers, employees, leaders, managers, investors and vendors. Success in your business depends on the people you encounter. How you treat those people will have a significant impact on your bottom line.
Here are some ways to help people in your business and show them that they matter:
1. Deliver incredible customer service.
2. Give a single parent the afternoon off.
3. Pay someone more than you need to.
4. Send a hand-written note to an employee acknowledging a job well done.
5. Loan a subordinate a book that you’ve found to be helpful.
6. Tell someone struggling “I want to help you succeed,” and mean it.
7. Stop by and talk, without an agenda.
8. Share a resource that you’ve found useful.
9. Create a safe space for openness and honesty.
10. Let people tell you how they feel, and listen without interrupting.
I encourage you to think about the people that you impact, the lives that you touch and the positive changes that you can make. These are the true signs of leadership and in my opinion are more important than the money that you make, the position that you hold or the size of your office.
You can do good while you’re doing well. It may require you to think of new ways to do it, but when you focus on helping others, you’ll succeed in ways beyond measure.
Sunday, December 26, 2010
Combined cycle power plant

combined cycle power plant is more efficient than a conventional power plant because it uses a higher proportion of the energy that the fuel produces when it burns.
In a combined cycle power plant (CCPP), or combined cycle gas turbine (CCGT) plant, a gas turbine generates electricity and the waste heat is used to make steam to generate additional electricity via a steam turbine; this last step enhances the efficiency of electricity generation. Most new gas power plants in North America and Europe are of these types.
The Integrated Gasification Combined Cycle, or IGCC, is a process that turns coal into gas known as synthesis gas (syngas) before it is burnt. The impurities in the syngas, like CO2, can also be removed before it is burnt.
The syngas is often used to power a gas turbine generator for electricity whose waste heat is passed to a steam turbine system. If CO2 is removed before burning (pre-combustion) it can be stored in deep geological formations..
Labels:
Combined cycle power plant
Saturday, December 25, 2010
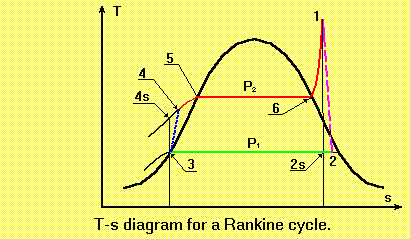
Steam Turbine
Steam turbines are devices which convert the energy stored in steam into rotational mechanical energy. These machines are widely used for the generation of electricity in a number of different cycles, such as:
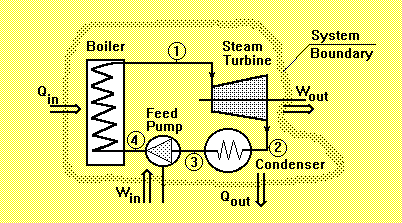
Consider the steam turbine shown in the cycle above. The output power of the turbine at steady flow condition is:
The efficiency of the steam turbines are often described by the isentropic efficiency for expansion process. The presence of water droplets in the steam will reduce the efficiency of the turbine and cause physical erosion of the blades. Therefore the dryness fraction of the steam at the outlet of the turbine should not be less than 0.9.
- Rankine cycle
- Reheat cycle
- Regenerative cycle
- Combined cycle
Consider the steam turbine shown in the cycle above. The output power of the turbine at steady flow condition is:
P = m (h1-h2)where m is the mass flow of the steam through the turbine and h1 and h2 are specific enthalpy of the steam at inlet respective outlet of the turbine.
The efficiency of the steam turbines are often described by the isentropic efficiency for expansion process. The presence of water droplets in the steam will reduce the efficiency of the turbine and cause physical erosion of the blades. Therefore the dryness fraction of the steam at the outlet of the turbine should not be less than 0.9.
Labels:
Steam Turbine
Heat Engine
Heat engine is defined as a device that converts heat energy into mechanical energy or more exactly a system which operates continuously and only heatand work may pass across its boundaries.
The operation of a heat engine can best be represented by a thermodynamic cycle. Some examples are: Otto, Diesel, Brayton, Stirling and Rankine cycles.
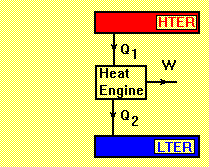
LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
A forward heat engine has a positive work output such as Rankine or Brayton cycle. Applying the first law of thermodynamics to the cycle gives:
The second law of thermodynamics states that the thermal efficiency of the cycle, , has an upper limit (the thermal efficiency of the Carnot cycle), i.e.
, has an upper limit (the thermal efficiency of the Carnot cycle), i.e.

It can be shown that:
which means that it is impossible to convert the whole heat input to work and
which means that a minimum of heat supply to the cold reservoir is necessary.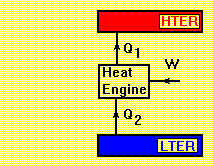
LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
A reverse heat engine has a positive work input such as heat pump and refrigerator. Applying the first law of thermodynamics to the cycle gives:
which means that the coefficient of performance for a heat pump is greater than unity.
The operation of a heat engine can best be represented by a thermodynamic cycle. Some examples are: Otto, Diesel, Brayton, Stirling and Rankine cycles.
Forward Heat Engine
LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
A forward heat engine has a positive work output such as Rankine or Brayton cycle. Applying the first law of thermodynamics to the cycle gives:
Q1 - Q2 - W = 0
The second law of thermodynamics states that the thermal efficiency of the cycle,
It can be shown that:
Q1 > W
which means that it is impossible to convert the whole heat input to work and
Q2 > 0
which means that a minimum of heat supply to the cold reservoir is necessary.
Reverse Heat Engine
LTER= Low Temperature Energy Reservoir
HTER= High Temperature Energy Reservoir
A reverse heat engine has a positive work input such as heat pump and refrigerator. Applying the first law of thermodynamics to the cycle gives:
- Q1 + Q2 + W = 0In case of a reverse heat engine the second law of thermodynamics is as follows: It is impossible to transfer heat from a cooler body to a hotter body without any work input i.e.
W > 0
which means that the coefficient of performance for a heat pump is greater than unity.
Labels:
Heat Engine
Friday, December 24, 2010
Fundamentals of Thermodynamics
- Introduction, laws of thermodynamics, notation.
- Work and processes.
- First and second laws.
- Gibbsian equations, chemical potential.
- Mathematical methods, Legendre transforms.
- Partial derivative game.
- Process evaluation. Residual functions.
- Residual functions and example problems.
- Introduction to statistical mechanics and quantum mechanics.
- Quantum mechanics I.
- Quantum mechanics II.
- Check out this link for a description of the rotational energy of a molecule.
- Statistics and ensembles.
- Ensembles and partition functions.
- Semi-classical partition function.
- Properties of ideal gases.
- Properties of ideal gases, examples.
- Chemical equilibria: ideal and real fluids.
- Pair potentials and nonideal behavior. Van der Waals partition functions for mixture, local compositions, activity coefficient models.
- Conformal solution theory.
- Conformal solution theory, pure fluids and mixtures.
- Ideal solutions and partial molar quantities.
- Partial molar properties and fugacity.
- Local composition models.
- Applet for plotting radial distribution functions.
- Molecular simulation code written as a Java Applet.
- Mixture thermodynamics calculations.
- Local compositions.

Labels:
Fundamentals of Thermodynamics
Thursday, December 23, 2010
Relation between PVT
Gas Laws:
Relationship between Pressure (P), Volume (V), Temperature (T) and quantity; Moles (n)
Boyle’s Law (PV = constant)
This is an inverse relationship: As volume decreases, pressure increases.
Charles’ Law: (V/T = constant)
This is an inverse relationship: As volume decreases, pressure increases.
Charles’ Law: (V/T = constant)
This is a direct relationship. As Temperature decreases, Volume decreases.
Avogadro’s Law: (V/n = constant)
This is a direct relationship. As the number of moles decreases, the volume decreases
Summary:
Combined Gas Law:
PV/nT = constant (T in Kelvins)
P1V1/n1T1 = P2V2/n2T2
Ideal gas Law:
PV = nRT
R = .0821L atm/mol K
Gay-Lussac’s/Avogadro’s Law of Combining Volumes
Equal volumes of any gases at the same temperature and pressure contain the same number of moles of
gas.
The coefficients of a balanced equation can be used to calculate relative volumes.
Standard Molar Volume: At standard temperature and pressure (STP = 1atm and 273.15K) 1 mole of any
ideal gas has a volume of 22.4L
Variations on the ideal gas law equation:
PV = mRT/M (m = sample mass, M = molar mass of the gas)
d = MP/RT (d = density of the gas in g/L)
Examples:
1. Calculate:
a. The new pressure in a closed container if a 5.0L volume of gas at 2.5atm has its volume increased to
7.5L.
b. The new volume of gas (at constant T and P) if 2.0mol of He in a 3.0L container has another 3.0mol of
He placed into the container.
Answers:
a. (5.0L)(2.5atm) = (7.5L)(P2)
P2 = 1.7atm
b. 3.0L/2.0mol = V2/5.0mol
V2 = 7.5L
2. When a rigid hollow sphere containing 680 L of helium gas is heated from 300.K to 600.K, the
pressure of the gas increases to 18atm. How many moles of helium does the sphere contain?
Answer:
n = PV/RT = (18atm)(680L)/(.0821)(600.K)
n = 248.48 = 250moles
3. A child has a lung capacity of 2.2 L. How many grams of air do her lungs hold at a pressure of 1.0
atm and a normal body temperature of 37
o
C? Assume a “formula mass” of 29g/mol for air.
Answer:
m = MPV/RT = (29g/mol)(1.0atm)(2.2L)/(.0821Latm/molK)(310.15K) = 2.5g
4. A gas with a volume of 300.mL at 150.
o
C is heated until its volume is 600.mL. What is the new
temperature of the gas if the pressure is unaltered?
Answer:
300mL/423.15K = 600mL/T2
T2 = 846K = 573
o
C5. Calculate the number of liters occupied, at STP.
a. 0.350 mol O2
b. 63.5g He
Answers:
a. 0.350mol (22.4L/mol) = 7.84L
b. (63.5g)(1mol/4.003g)(22.4L/1mol) = 355L
6. Determine the molar mass of a gas for which a 2.5g sample of that gas occupies a volume of 3.0L at
STP.
Answer:
M =(2.5g)(.0821)(273.15K)/(3.0L)(1atm) =18.7g/mol
or
(2.5g)/(3.0L/22.4L/mol) = 18.7g/mol
7. Find the density of fluorine gas (g/L) at 700torr and 50
o
C.
Answer:
d = MP/RT
= (38.00g/mol)(700/760) / (.0821)(50+273.15) = 1.32g/L
8. For the equation
Ag2S(s)
+ H2(g) → Ag(s)
+ H2S(g)
How many Liters of H2S can be produced from 15.0g of Ag2S and 1.00L of H2(g)
if the reaction occurs at
STP?
Answer:
Ag2S(s)
+ H2(g) → 2Ag(s)
+ H2S(g)
mol Ag2S = 15.0g (1mol/247.8g) = .0605mol
mol H2 = (1.00L)(1mol / 22.4L) = .0446mol
Hydrogen gas limits
0446mol H2 (1mol H2S / 1mol H2) = .0446mol H2S.
V = .0446mol (22.4L/mol) = 1.0L
(Note that the last two steps aren’t really necessary because of the 1:1 mole ratio between H2S and H2.)
Relationship between Pressure (P), Volume (V), Temperature (T) and quantity; Moles (n)
Boyle’s Law (PV = constant)
This is an inverse relationship: As volume decreases, pressure increases.
Charles’ Law: (V/T = constant)
This is an inverse relationship: As volume decreases, pressure increases.
Charles’ Law: (V/T = constant)
This is a direct relationship. As Temperature decreases, Volume decreases.
Avogadro’s Law: (V/n = constant)
This is a direct relationship. As the number of moles decreases, the volume decreases
Summary:
Combined Gas Law:
PV/nT = constant (T in Kelvins)
P1V1/n1T1 = P2V2/n2T2
Ideal gas Law:
PV = nRT
R = .0821L atm/mol K
Gay-Lussac’s/Avogadro’s Law of Combining Volumes
Equal volumes of any gases at the same temperature and pressure contain the same number of moles of
gas.
The coefficients of a balanced equation can be used to calculate relative volumes.
Standard Molar Volume: At standard temperature and pressure (STP = 1atm and 273.15K) 1 mole of any
ideal gas has a volume of 22.4L
Variations on the ideal gas law equation:
PV = mRT/M (m = sample mass, M = molar mass of the gas)
d = MP/RT (d = density of the gas in g/L)
Examples:
1. Calculate:
a. The new pressure in a closed container if a 5.0L volume of gas at 2.5atm has its volume increased to
7.5L.
b. The new volume of gas (at constant T and P) if 2.0mol of He in a 3.0L container has another 3.0mol of
He placed into the container.
Answers:
a. (5.0L)(2.5atm) = (7.5L)(P2)
P2 = 1.7atm
b. 3.0L/2.0mol = V2/5.0mol
V2 = 7.5L
2. When a rigid hollow sphere containing 680 L of helium gas is heated from 300.K to 600.K, the
pressure of the gas increases to 18atm. How many moles of helium does the sphere contain?
Answer:
n = PV/RT = (18atm)(680L)/(.0821)(600.K)
n = 248.48 = 250moles
3. A child has a lung capacity of 2.2 L. How many grams of air do her lungs hold at a pressure of 1.0
atm and a normal body temperature of 37
o
C? Assume a “formula mass” of 29g/mol for air.
Answer:
m = MPV/RT = (29g/mol)(1.0atm)(2.2L)/(.0821Latm/molK)(310.15K) = 2.5g
4. A gas with a volume of 300.mL at 150.
o
C is heated until its volume is 600.mL. What is the new
temperature of the gas if the pressure is unaltered?
Answer:
300mL/423.15K = 600mL/T2
T2 = 846K = 573
o
C5. Calculate the number of liters occupied, at STP.
a. 0.350 mol O2
b. 63.5g He
Answers:
a. 0.350mol (22.4L/mol) = 7.84L
b. (63.5g)(1mol/4.003g)(22.4L/1mol) = 355L
6. Determine the molar mass of a gas for which a 2.5g sample of that gas occupies a volume of 3.0L at
STP.
Answer:
M =(2.5g)(.0821)(273.15K)/(3.0L)(1atm) =18.7g/mol
or
(2.5g)/(3.0L/22.4L/mol) = 18.7g/mol
7. Find the density of fluorine gas (g/L) at 700torr and 50
o
C.
Answer:
d = MP/RT
= (38.00g/mol)(700/760) / (.0821)(50+273.15) = 1.32g/L
8. For the equation
Ag2S(s)
+ H2(g) → Ag(s)
+ H2S(g)
How many Liters of H2S can be produced from 15.0g of Ag2S and 1.00L of H2(g)
if the reaction occurs at
STP?
Answer:
Ag2S(s)
+ H2(g) → 2Ag(s)
+ H2S(g)
mol Ag2S = 15.0g (1mol/247.8g) = .0605mol
mol H2 = (1.00L)(1mol / 22.4L) = .0446mol
Hydrogen gas limits
0446mol H2 (1mol H2S / 1mol H2) = .0446mol H2S.
V = .0446mol (22.4L/mol) = 1.0L
(Note that the last two steps aren’t really necessary because of the 1:1 mole ratio between H2S and H2.)
Labels:
Relation between PVT
Subscribe to:
Posts (Atom)







